We’re used to our universe behaving in a certain way. There is a natural progression of events, of causes and effects. Living creatures and physical objects behave according to the laws of motion, quantum mechanics and relativity. But there is something else at work here too: a natural phenomenon that affects the fate of everything in our universe. We are capable of building amazing and complex structures, but everything that we create eventually decays and falls apart. Living organisms age and deteriorate over time.
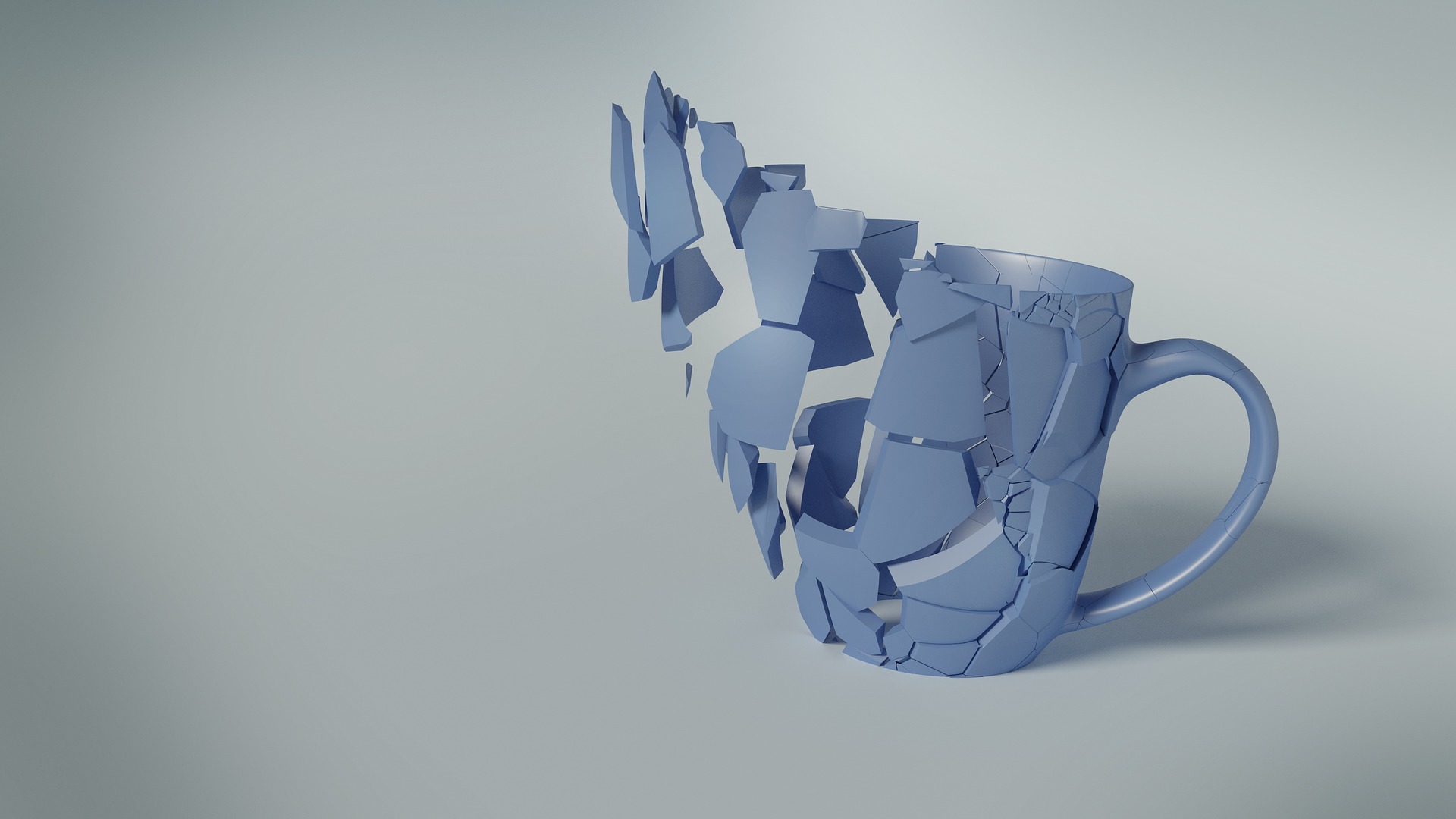
A man preparing a delicious snack becomes distracted. A glass of milk falls and breaks. We’ve all seen this before. But why does this happen? Well aside from carelessness that is, why don’t we ever see the opposite? Why don’t we ever see shards of broken glass fuse together and combine with liquid to create a full unbroken cup? According to the laws of physics, this is technically possible. But we all know from experience that this isn’t the way things work. In fact every glass cup ever made will ultimately share the same shattered broken fate, whether it’s in a dumpster, recycling plant or on your kitchen floor. It’s the natural way of our universe and it’s all because of something called entropy.
What is entropy?
There are several different definitions, entropy is first of all a measure of disorder, it’s a measure of how many ways you can reorganize a given system.
The entropy of an ideal gas for example can be expressed with this equation: S=KBlnW; where S equals entropy KB is the Boltzmann constant, which equal 1.38062×10-23Joules/Kelvin. This equation knows as Boltzmann’s entropy formula.
Let’s just say you’re playing a game of pool; a typical game begins with the balls arranged in a highly ordered configuration. This is a low entropy state. As the game begins, however, the balls are quickly scattered. The disorder of the table increases, and the number of possible configurations, Therefore the entropy of the pool table has increased.

Now try to the opposite with the single shot, put them back in the order!
Technically it’s not an impossible task. But it’s extremely unlikely and something we probably won’t ever see in our lifetime. This natural tendency of entropy to increase over time is described by the second law of thermodynamics.
The second law of thermodynamics
The second law of thermodynamics will tell you that the entropy of an isolated system cannot decrease as a function of time. So as time goes, it whether remains the same or increase. A perfect example of this is life; a living organism is a highly ordered low entropy system. And life on this planet at least can pretty much pop up anywhere we look, even from a rough chaotic environment. But this order does not come without a price! It takes a lot of energy to lower the entropy of a system. In the case of plant life most of this energy comes from the Sun. And the Sun is constantly radiating energy into space, increasing the entropy of our entire solar system.

A hot cup of coffee has higher entropy than a cold cup of coffee, because hot water molecules have more movement than cold water molecules, and are therefore more disordered. Ice has even less entropy because of the restricted movement of water molecules in a solid formation.
We all know that a hot cup of coffee will cool over time, and it’s delicious. But be careful that as it cools, its entropy will actually decrease. However a coffee cup is not an isolated system and the only way it can lower its entropy is by releasing its heat into the room, thereby increasing the total entropy of the surrounding environment.
Getting a haircut is another example of taking a disordered system and transforming it into an ordered system; a sexy ordered system. However, even though the entropy may be lower on your head and face, according to the second law of thermodynamics, the overall entropy of the entire system must have increased. The barber burned calories to operate the scissors. Fossil fuels were incinerated to provide electricity for the Clippers. And the hair that was removed was chaotically dispersed across the floor. Only to be swept up requiring even more energy. But why is this? Why must we live in a universe where disorder is constantly growing, and order is so hard to come by?
The Big Bang
It’s not something that follows from the basic laws of physics; it’s really a consequence of statistics. There are many more high entropy states available than low entropy states. There is strong evidence that the universe is started with what is now known as the Big Bang.
The Big Bang was a state of very low entropy. The entropy has since increased a lot. And there is no evidence that they will start decreasing anytime soon.
Because there are so many more disordered States than ordered states, the tendency of the universe can move towards disorder is just a matter of probability. It is a process that began with the Big Bang, and it has been going on ever since. As much as we try to create and sustain order in our lives, we are all bound to the second law of thermodynamics. The increasing disorder and decay of our universe is inevitable. But if ordered states are so improbably, it’s amazing that we’re even here at all. We should be thankful! If things get chaotic from time to time well that’s just the way of the world.
Read also: What Probability Means, How Is It Used In Real Life?






